Transforming Transplant Outcomes
Immune-Edited Organs: Transplants Accepted Naturally, Without Lifelong Immunosuppression
Innovative Transplant Solutions
Enhancing quality of life for transplant recipients through advanced immunomodulation and machine perfusion technologies.
Immunedit™ Platform
Modulating the immune profiles of donor organs through the insertion or deletion of immunomodulatory molecules via mRNA/siRNA transfer.
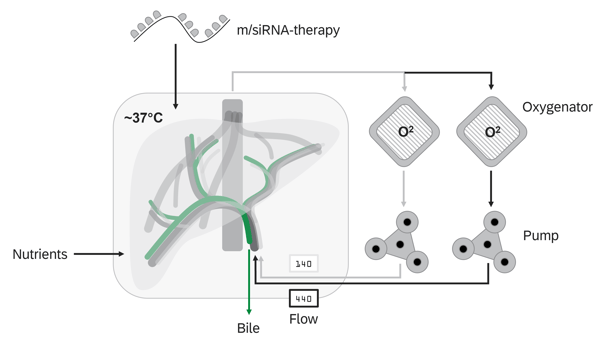
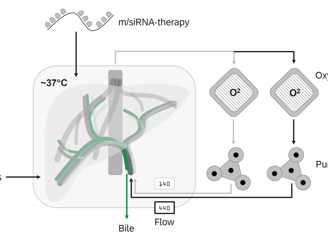
Normothermic Machine Perfusion
NMP technology facilitates the ex vivo treatment of donor organs in a near-physiological state for extended periods
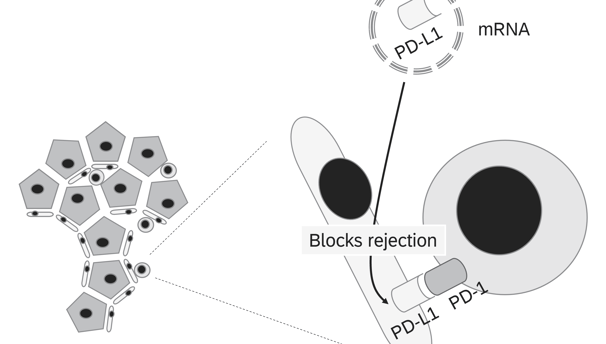
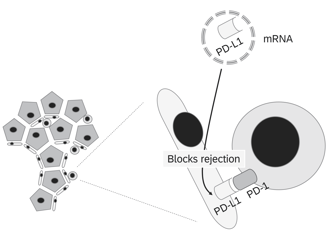
Potential targets
To induce local tolerance of the graft, checkpoint molecules that negatively regulate the immune response can be modulated either by mRNA insertion (to express these molecules) or siRNA silencing (to knock down or reduce expression of molecules that trigger immune activation). Here are some key checkpoint molecules involved in immune regulation:
Checkpoint Molecules for mRNA Insertion:
These molecules can be inserted into the donor organ during normothermic machine perfusion to promote immune tolerance by enhancing immune suppression.
1. PD-L1 (Programmed Death-Ligand 1)
• Function: Binds to PD-1 receptors on T cells, suppressing their activation and promoting immune tolerance.
• Goal: Upregulating PD-L1 locally on the graft can inhibit T cell-mediated rejection and promote tolerance.
2. CTLA-4 (Cytotoxic T-Lymphocyte-Associated Protein 4)
• Function: A key negative regulator of immune responses, competing with CD28 for binding to B7 ligands on antigen-presenting cells (APCs), thus dampening T cell activation.
• Goal: Overexpression of CTLA-4 could enhance immune suppression, preventing T cell activation at the site of the graft.
3. IDO (Indoleamine 2,3-Dioxygenase)
• Function: Metabolizes tryptophan, leading to the depletion of tryptophan in the local microenvironment, which inhibits T cell proliferation and promotes regulatory T cell (Treg) function.
• Goal: Increasing IDO expression could help reduce the immune response and foster an immunosuppressive microenvironment.
4. TGF-β (Transforming Growth Factor Beta)
• Function: A potent immunosuppressive cytokine that promotes the generation of regulatory T cells (Tregs) and suppresses effector T cell activity.
• Goal: Upregulation of TGF-β could promote immune tolerance through the generation of Tregs at the graft site.
5. IL-10 (Interleukin-10)
• Function: An anti-inflammatory cytokine that inhibits the activity of various immune cells, including T cells, macrophages, and dendritic cells.
• Goal: Overexpression of IL-10 in the graft can create an anti-inflammatory environment conducive to tolerance.
Checkpoint Molecules for siRNA Silencing:
These molecules are typically associated with immune activation and can be silenced to prevent the immune system from attacking the graft.
1. CD40
• Function: CD40 is expressed on antigen-presenting cells and engages with CD40L on T cells to promote immune activation.
• Goal: Silencing CD40 in the donor tissue can prevent the activation of T cells and reduce graft rejection.
2. CD86 (B7-2) and CD80 (B7-1)
• Function: Co-stimulatory molecules that interact with CD28 on T cells, leading to their activation.
• Goal: Knockdown of CD86 or CD80 can reduce co-stimulation and T cell activation, promoting tolerance.
3. ICAM-1 (Intercellular Adhesion Molecule 1)
• Function: Facilitates the adhesion of T cells to endothelial cells and antigen-presenting cells, promoting immune cell infiltration and activation.
• Goal: Silencing ICAM-1 can reduce immune cell recruitment to the graft site, minimizing inflammation and immune attack.
4. LFA-1 (Lymphocyte Function-Associated Antigen 1)
• Function: A molecule on T cells that interacts with ICAM-1 to facilitate T cell adhesion and migration to sites of inflammation.
• Goal: Silencing LFA-1 can prevent T cell infiltration into the graft, promoting local immune tolerance.
5. CXCL10 (C-X-C Motif Chemokine Ligand 10)
• Function: A chemokine that recruits T cells to sites of inflammation.
• Goal: Silencing CXCL10 can reduce T cell trafficking to the graft and lower immune-mediated damage.
6. TLR (Toll-like Receptors)
• Function: TLRs recognize danger signals and pathogens, leading to immune activation.
• Goal: Silencing key TLRs (e.g., TLR4) in the graft can reduce the inflammatory response and immune activation.
7. NLRP3 (NOD-like Receptor Pyrin Domain-Containing 3)
• Function: A key component of the inflammasome, which activates inflammatory pathways and promotes immune responses.
• Goal: Silencing NLRP3 can inhibit inflammasome activation, reducing graft inflammation and promoting tolerance.
Combinatorial Approaches:
• A combination of mRNA insertion and siRNA silencing can be used to both enhance local immune suppression (e.g., upregulating PD-L1 or TGF-β) and prevent immune activation (e.g., silencing CD40 or ICAM-1).
• Personalized strategies could be developed based on the specific immune profile of the recipient, using a tailored balance of checkpoint molecule modulation.
By leveraging these checkpoint molecules, Novagraft can aim to create a local immune-tolerant environment in donor organs, reducing the need for systemic immunosuppression and its associated side effects.
